Submissions
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.- The submission has not been previously published, nor is it before another journal for consideration (or an explanation has been provided in Comments to the Editor).
- The submission file is in OpenOffice, Microsoft Word, or RTF document file format.
- Where available, URLs for the references have been provided.
- The text employs italics, rather than underlining (except with URL addresses); and all illustrations, figures, and tables are placed within the text at the appropriate points, rather than at the end,
- The text adheres to the stylistic and bibliographic requirements outlined in the Author Guidelines.
- This work
Author Guidelines
Publication Standards of the Revista Médica del Uruguay
Scope and editorial policy
The Uruguayan Medical Journal (RMU) is the official scientific publication of the Uruguayan Medical Union (SMU). It publishes articles referring to topics in the field of medicine and works under a double-blind arbitration system. The RMU adheres to the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals, published by The International Committee of Medical Journal Editors.
The RMU is an open access journal. Adopts the Creative Commons license known as Attribution 4.0 International (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/deed.es. This license allows the user to share (copy and redistribute the material in any medium or format).
Opinions or statements expressed in the journal reflect the views of the authors; they do not represent the official opinion of the Editorial Board unless expressly stated so.
Admission process
The manuscript sent for publication will be examined in the first instance by the Editorial Board. It will evaluate the form and content of the presentation. If accepted, it will be submitted to arbitration by two expert reviewers appointed by the Council. Once the opinion of the reviewers has been received, it will be evaluated again by the Editorial Board, from which it may result:
a) acceptance of the manuscript without modifications;
b) non-acceptance;
c) the need for review by the authors, taking into account the suggestions for modifications made by the reviewers, and sending the corrected version for further consideration by the Editorial Board.
The arbitrators do not know the identity of the authors nor are they informed who carried out the arbitration. Likewise, in the publication process, the manuscripts will be subject to style correction, so they may be modified by the editorial staff of the journal. In all cases, it will be communicated to the authors. The evaluation process of a manuscript that includes a necessary review, as indicated in paragraph c) above, may take a time that should not exceed six to eight weeks. If the author does not comply with the deadlines requested by the RMU, his work will be withdrawn or may be considered a new article and be subjected to full arbitration again.
The author will retain the rights of him as author. The author responsible for the correspondence will indicate by letter or email the acceptance of the publication conditions, signing and dating it, after his work has been definitively accepted for publication, accepting the changes introduced by the Editorial Board, if any. . He must declare that he agrees with the CC-BY license that governs the magazine and that authorizes the RMU to publish and disseminate his work (work).
General order requirements
It is essential that the presentation of the work is done in accordance with the rules detailed below. Failing that, it will be a reason for non-acceptance or delay in publication.
Shipment. The articles will be sent by email to the address secretariarmu@gmail.com
Presentation letter
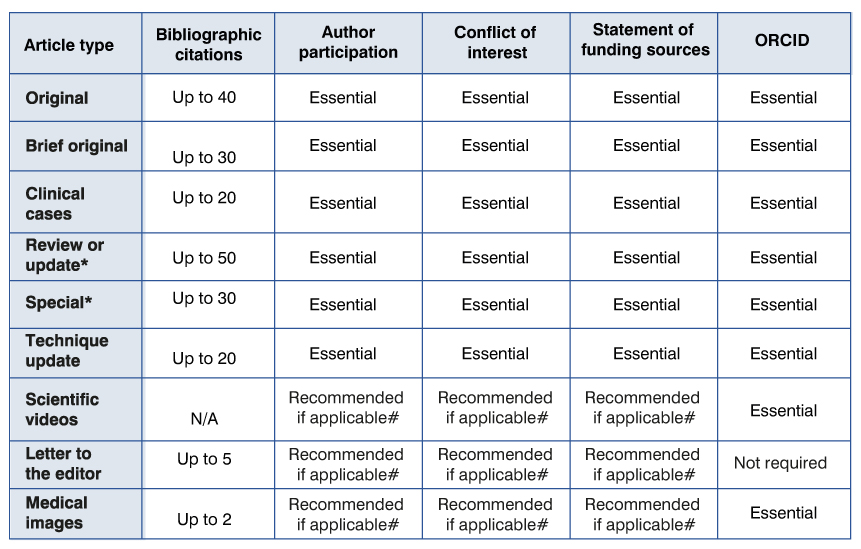
All papers must be accompanied by a cover letter, signed by all the authors, (in Word or similar format and in pdf or jpg for the purpose of including signatures) specifying:
a) title of the work and authors;
b) suggested location within the sections that comprise the magazine;
c) declaration that all the authors know, participate and agree with the content of the manuscript;
d) declaration that the article is not simultaneously presented in other media nor has it been previously published. Failing that, this last circumstance must be specified to the editor for its consideration;
e) statement that the work has been prepared in compliance with international and national recommendations on clinical research, ethical standards on animal research or those that correspond, depending on the content of the work (see specific section below);
f) name, telephone and email of the author in charge of the correspondence with the RMU, for the purposes of communication in the progress of the process.
g) that he knows and agrees with the RMU Publication Regulations;
h) declaration of conflict of interest;
i) ORCID: the ORCID number of each author must be detailed.
j) participation of each author. At the end of the cover letter, the degree of participation of each author in the article that is sent for publication should be indicated: conception, design, execution, analysis, interpretation of the results, writing, critical review. The roles of the authors in contributing to the research results should be indicated according to the CRediT Taxonomy (available at https://credit.niso.org/). It is recalled that it is necessary to have participated in at least two of these stages in order to be considered the author of the manuscript. Other contributions may be included in the acknowledgments section.
Redundant or duplicate publication
The journal will not accept articles already published in other media, unless the authors declare and expressly request, with a statement of reasons, prior consideration by the Editorial Board and formal agreement between the editors of both journals. The absence of a statement of the fact in the cover letter, or its alteration, may be grounds for immediate rejection of the article.
Ethics
All research involving human beings must respect international and national ethical standards. When they involve human beings, they must be governed by the provisions of Decree 158/019, without prejudice to other applicable regulations.
In this sense, in the body of the manuscript, in the material and methods section, a section or a paragraph must be made that establishes that the research has been approved by a Research Ethics Committee duly accredited before the National Ethics Commission of the Investigation, in accordance with the provisions of the aforementioned decree. It must be established which Committee approved the study, the date of approval and number, if applicable. This statement, inserted in the text of the article, has the value of a sworn statement by the authors. The magazine disclaims all responsibility, falling on the authors. This requirement is considered essential for the consideration of the articles; its absence will determine that the article cannot be considered by the Editorial Board to go to peer review.
For the publication of clinical cases, including images, the patient's consent will be required, which must be expressly stated in the body of the manuscript. If the consent of the patients cannot be obtained, the consent of immediate family members or legal representatives must be obtained. Failing that, request endorsement from a duly accredited Research Ethics Committee. The declaration of consent or endorsement to publish the clinical case has the value of an affidavit on the part of the authors. The magazine disclaims all responsibility, falling on the authors. This requirement is considered essential for the consideration of the articles; its absence will determine that the article cannot be considered by the Editorial Board to go to peer review.
Conflict of interest
Manuscripts must include, preferably on the first or second page, a statement on the existence or not of conflict of interest. An actual or potential conflict of interest exists when an author (or the institution to which the author belongs) has personal or financial relationships (also known as dual commitments, competing interests, or conflict of loyalties) that may inadequate (bias) in their actions. These relationships range from those whose potential is negligible to those with sufficient potential to influence scientific judgments. The possibility of a conflict of interest may exist whether or not a person believes that the relationship affects his or her scientific judgment. Financial relationships—such as employment, consultancies, stock ownership, fees, reports, and expert opinions—are the easiest conflicts of interest to spot. However, conflicts can occur for other reasons, such as personal relationships or academic rivalry, among other causes. In case of acceptance of the manuscript, the declaration will be published at the bottom.
Costs: The RMU does not charge any fees for submitting or processing articles.
Manuscript submission formalities
Articles will be written in Spanish, in clear and concise language. They will be presented in A4 or letter format, double spaced, preferably in Word or similar language, font size 12, with 2.5 cm margins, independently including figures and tables, all with the appropriate electronic support. The tables and graphs must be in Excel format and the figures with a resolution of 300 dpi.
The RMU consists of the following sections:
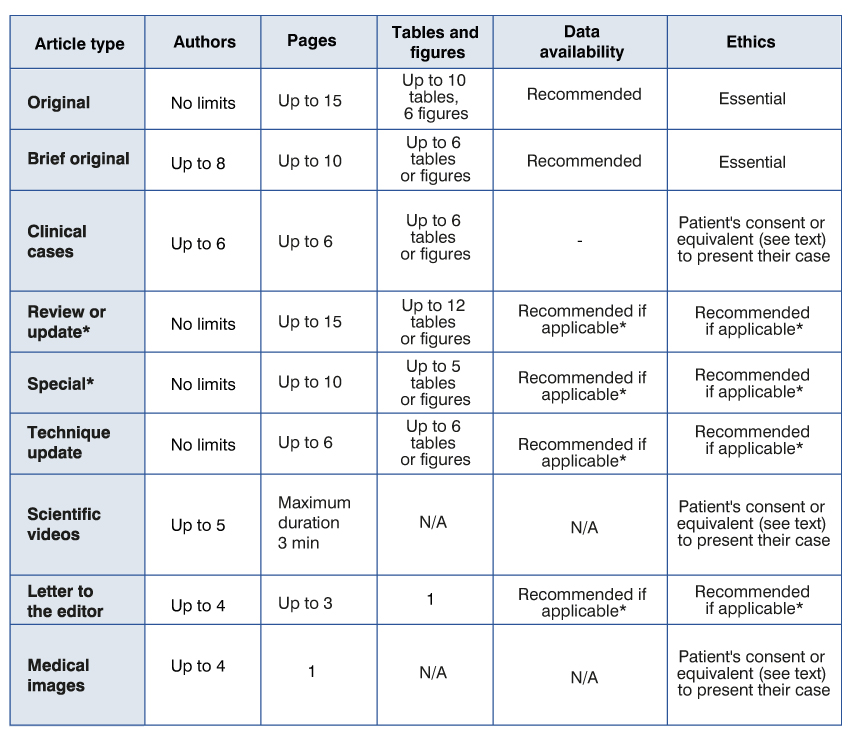
- Original Articles Section
This section is intended for qualitative or quantitative research articles. Articles must be presented according to the following scheme. Title page. Must include:
- Title of the article written concisely but informatively.
- Full name of each author. The names of the authors should appear only on the title page. The ORCID number of each author must be detailed.
- Teaching, scientific or professional positions they hold, name of the department, institution or dependency where they work.
- Name of the department and institution.
- Name and email of the author responsible for the correspondence of the manuscript.
- The source of support in the form of grants, equipment, drugs or all of them if applicable.
- Abbreviated title with no more than 40 characters.
- Declaration of conflict of interest.
- All original articles must include a section on data availability. It must be reported if the data is available and in which case where and how to access it. Authors are recommended to use the Scielo data repository: https://data.scielo.org/ In any case, the data must maintain absolute confidentiality. Otherwise, the data must be declared as unavailable. The following aphorism(s) is/are advised: "The data set that supports the results of this study are available at..." Otherwise, you should include a sentence within the article stating the following: "The data set that supports the results of this study are not available".
- The roles of the authors in contributing to the research results should be indicated according to the CRediT Taxonomy (available at https://credit.niso.org/).
Abstracts and keywords
All papers submitted, except Letters to the Editor and Editorial Comments, must include a summary of what has been stated, where the reader is allowed to enter the topic, it must not have more than 300 words, and it must highlight the importance of the topic. It must contain: introduction, objectives, method, main results and conclusions. New and important aspects of the study and observations should be emphasized. The impersonal form must be used, omitting critical judgments or comments about the value of the item. Author citations and references to figures and tables will be avoided. Papers that do not meet these abstract writing requirements will not be accepted. All articles, regardless of the section to which they belong -except for the exceptions specified in these regulations- must include a summary with the characteristics described here.
Keywords: a maximum of five will be used. They will be placed after the abstract and must describe the content of the article and facilitate its inclusion in indexes. The author should determine them based, as far as possible, on the MeSH (Medical Subject Headings) descriptors of the Index Medicus or the DeCS (Health Descriptors) of BIREME (Regional Library of Medicine).
Text: no more than 15 pages. It will start on page 3. In general, although not necessarily, it will consist of the following sections: Introduction – Material and method – Results – Discussion – Conclusions. Very long articles may need subheadings to these sections in order to clarify their content.
Introduction: The nature, foundations and objectives of the study are clearly stated, giving an idea of its scope and importance, as well as its limitations. The objectives should be listed at the end of the introduction. The authors must carry out a review of the literature that allows updating knowledge only on matters that are directly and specifically related to the work in question. In all cases, the sources of information used must be mentioned. It is convenient to avoid excessive citations, previously submitting them to a selection that ensures unity and thematic coherence.
Material and method: the procedures used are described so that the reader can judge about the propriety of the methods, the degree of precision of the observations and the reproducibility of the study. Methods, apparatus (manufacturer's name and address in parentheses), and procedures are identified in detail, so as to allow reproduction of the results. References to established methods will be given, including in this case a brief description. New or substantially modified methods will be described, explaining the reasons for their use and evaluating their limitations. The chemical products and drugs used are mentioned by the active ingredient, including dosage and method of administration.
Ethical standards: it must be specified in accordance with what was previously established. Appropriate approval must be included. In the case of experiments on animals, indicate whether the standards of the institution or the National Research Council were followed, or any national law on the care and use of laboratory animals.
Statistics: describe the statistical methods in sufficient detail to allow the prepared reader, with access to the original data, to verify the results presented. Quantify the findings, whenever possible, and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). The eligibility of experimental subjects should be discussed. Details of randomization should be given. The methods and success of any kind of blind-watching technique should be described. Inform about the complications of the treatment. Specify the number of observations. Mention cases lost to observation (such as dropouts in a clinical trial). References for study design and statistical methods should refer, where possible, to standard papers (with page names), rather than to papers where the designs or methods were originally published. Specify any general purpose software used.
Results: it is the rigorous report of experimental observation. It must be presented in a clear, concise and logical manner, using tables, statistics, figures and other illustrations that allow a better interpretation of the facts to be demonstrated. They must be adjusted to the objectives set out in the Introduction.
Discussion: a judgment is opened on the results obtained, their suitability and limitations are explained, discussed and pointed out, comparing them with those of other authors. It must be shown how the data obtained allow or not to meet the proposed research objectives.
Conclusions: observations or important contributions of the work are highlighted, which must be fully supported by the results and be a response to the objectives of the research.
Thanks: they are addressed only to those people who have contributed substantially to the study.
Bibliography
No more than 40 bibliographic citations. The bibliographical references will be numbered consecutively, in the order in which they appear mentioned in the text. References that are only cited in tables or figures should be numbered according to their appearance in the text. They will be written according to the form adopted by the United States National Library of Medicine, used in the Index Medicus. Journal titles will be abbreviated according to the style adopted by Index Medicus. For Latin American journals, the abbreviations of the Latin American Index Medicus will be used. The use of “abstracts”, unpublished observations and “personal communications” as references should be avoided. The author should check the references with the original publications.
a) Magazine articles. Essential elements: author or authors of the article. Title of the same. Abbreviated title of the journal, year of publication; volume, number: pages. Up to six authors will be mentioned. When the article has seven or more, the first six are mentioned, followed by the Latin expression et al. You can include the DOI.
Example: Reta G, Riva J, Arcos J, Cedrés G, Álvarez, Meerovich E, et al. Estudio de la mecánica ventilatoria en pacientes con enfermedad pulmonar obstructiva crónica. Rev Méd Urug 1992; 8(2):131-40.
b) Books and other monographs. Essential elements: author/s. Title: subtitle. Edition. Place of publication (city): publisher, year; pages or chapter and volume. Personal author: the last name of the author and the initial of the name are mentioned. In the case of several authors, they are all mentioned separated by a comma. The initial of the name does not have a point. Up to six authors will be mentioned. When the article has seven or more, the first six are mentioned, followed by the Latin expression et al.
Example: Lolas F, Quezada A. Pautas éticas de investigación en sujetos humanos: nuevas perspectivas. Santiago de Chile: PAHO, 2003.
Corporative author. It is the entity responsible for the work. It is mentioned in its original language, in developed form.
Example: World Health Organization; Uruguayan Medical Union. They are noted as they appear in the publication.
Edition: indicated in Arabic numerals, followed by the abbreviation ed. If it is a first edition, it should not be noted.
Imprint: place of publication (city): publisher (the main one is mentioned, eliminating words such as Company, Limited, and Sons, etc.) and year of publication.
Example: Mexico: Interamericana, 1976. Pages: They are mentioned with Arabic numerals and may include: total number of pages: 729 p., consulted pages: 724-729 (724-9), Volume: v.5.
c) Part or chapter of a book. The ordering of the bibliographic data is as follows:
The main entry is made by the author(s) of the chapter, followed by the title of the chapter or part and then the full reference of the book, preceded by the expression In:
Example: Weinstein L, Swartz MN. Pathogenetic properties of invading microorganisms. In: Sodeman WA Jr, Sodeman WA, eds. Pathologic physiology: mechanisms of disease. Philadelphia: WB Saunders, 1974:457-72.
d) Congresses, Conferences, Meetings. Enter the author(s) of the paper or proceedings. Title of the paper or proceedings. In: Editors or organization responsible for the congress. Title of the congress or conference. Conference date. Congress venue. Place of publication: publisher, year. Pages.
Example: Rice AS, Farquhar Smith WP, Bridges D, Brooks JW. Cannabinoids and pain. In: Dostorovsky JO, carr DB, Koltzenburg M, eds. Proceedings of the 10th World Congress on Pain; 2002 Aug 17-22; San Diego, CA. Seattle (WA): IASP Press, 2003:437-68.
e) Electronic media. Electronic media can be divided into two main types: “online media” (web sites, scientific journal articles on the Internet, etc.), and other media (books or articles on CD-ROMs, diskettes, magnetic tapes, software). When citing electronic media, five basic principles must be taken into account: accessibility, intellectual property, economy, standardization and transparency.
f) Online journal article: author/s. Article title. Abbreviated title of the journal, year of publication; Volume (number): pages. Available at: URL address. [Query: date of access day, month and year]. Or in the case of having a DOI, only that data is placed.
Example 1: Branquinho D, Freire P, Sofia C. NOD2 mutations and colorectal cancer: where do we stand? World J Gastrointest Surg 2016; 8(4):284-93. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4840167/. [Consultation: May 12, 2016].
Example 2: Motta I, Mancarella M, Marcon A, Vicenzi M, Cappellini MD. Management of age-associated medical complications in patients with β-thalassemia. Expert Rev Hematol 2020; 13(1):85-94. doi: 10.1080/17474086.2020.1686354.
g) If the author is not documented, the title becomes the first reference element.
h) CD-ROM (books): Format: author/s. Qualification. (CD ROM). Edition. Production place. Producer; Year. Title of the serial CD-ROM or database (if online).
i) Software: Format: title (medium). Version (resource type, floppy, CD-ROM, online). Production place. Producer, Year.
Example: Epi Info (computer program). Version Atlanta, GA: Centers for Disease Control and Prevention, 1994.
Boards. No more than 10 tables or 6 figures. They must be done on separate sheets, respecting double spacing, consecutively numbered with Arabic numerals and with a brief title. Each column should have a short or abbreviated heading. The explanatory notes will go to the bottom of the page, using the following symbols in the order indicated: *, †, ‡, §, ¶, ††, ‡‡, as well as the explanation of the unknown abbreviations used in each board. If data from other sources are used, acknowledgment and permission should be mentioned.
Photographs. No more than six and not printed in color. In JPG or TIF. They will be very clear, no larger than 20 by 25 cm. Letters, numbers and symbols will be large enough to be legible after reduction. The titles and detailed explanations will go separately, in the legends for illustrations. All illustrations must be numbered and referred to in the text. In the case of microphotographs, the technique used will be indicated, as well as the scale. The symbols and letters must contrast with the background. In the case of sending illustrations or color photographs, the expenses will be borne by the author, unless the journal considers it essential to include them in color and can finance them.
Illustration legends. Legends should be double-spaced, each one on a separate page, with the number corresponding to the illustration. When symbols, numbers or letters are used to identify part of the illustration, it must be clearly explained in the legend.
Measurement units. Measurements of length, height, weight, and volume must be reported in metric units (meter, kilogram, liter) or their decimal multiples. Temperatures should be recorded in degrees Celsius. Blood pressure should be given in millimeters of mercury. In hematological and biochemical measurements, the metric system must be used according to the International System of Units (SI). Publishers may request that alternative or non-SI units be added by the author prior to publication.
Abbreviations and acronyms. Use only the standard abbreviation. Avoid abbreviations in the title and abstract. The full term that is represented by an abbreviation or acronym must precede its first use in the text, unless it is a standard unit of measure.
- Brief Original Articles Section
This section is intended for the publication of qualitative or quantitative research. It will follow the general scheme of presentation and structure of the Original Articles. Its maximum length will be ten double-spaced pages with font size 12. It may have up to 30 bibliographical citations that will fully conform to what was previously described. It may have up to six figures or tables. The number of authors may not exceed eight. They should include a section on data availability. It must be reported if the data is available and in which case where and how to access it. It can be a link to a database. The data must retain absolute confidentiality. Otherwise, the data must be declared as unavailable. The following aphorism(s) is/are advised: "The data set that supports the results of this study are available at..." Otherwise, you should include a sentence within the article stating the following: "The data set that supports the results of this study are not available".
- Clinical Cases Section
This section will be intended for the presentation of clinical cases or series of cases whose exceptional presentation deserves, in the opinion of the authors, to be communicated to the medical staff. It goes without saying that they must present the essential facts of the clinical case. The discussion and bibliography will be short and concise. No more than six authors should appear. The length of the text will not be greater than six double-spaced pages with font size 12. No more than six figures or tables will be included. It should be specified that the consent of the patient or family member was obtained for the publication of the clinical case.
- Revision or Update Works and Updates Section
This section is intended for the publication of reviews or updates on relevant or specific topics of medical practice. The revisions may be requested by the Editorial Board or suggested by the authors. The authors, prior to submitting the manuscript, should consult the Editorial Board about the relevance of such submission. The Editorial Board will observe the relevance of the topic and the experience of the authors in the matter. After obtaining the endorsement for sending the manuscript, it will enter the editorial process, going, in the first instance, to peer review. Reviews that have not entered under this procedure will not be considered.
The methodology of the review should be explained. In the case of being a systematic review, the sources consulted (for example: SciELO, Medline, Index Medicus, others), in which language it was carried out and which years it covers. Regardless of the type of review –systematic, exhaustive, descriptive, etc–, it is recommended that the article follow the following structure: Introduction, Methodology, Development and Discussion, Conclusions.
The maximum length will be 15 pages in Word or similar language, font size 12, double spaced, with a maximum of 12 figures or tables. The Bibliography will be placed in order of appearance according to what is indicated in the Original Articles section and may not exceed 50 bibliographic citations.
- Special Articles Section
This section is intended for the publication of articles that do not meet the characteristics described in the preceding sections. By way of example, opinion articles, history of medicine, bioethics, medical education, among others, may be included in this section. Opinion articles will be reserved for authorities on certain specific topics to issue their views on matters considered of special interest to the medical community. In the case of articles suggested by the authors, prior to submitting the manuscript, they should consult the Editorial Board about the relevance of such submission. The Editorial Board will observe the relevance of the topic and the experience of the authors in the matter. After obtaining the endorsement for sending the manuscript, it will enter the editorial process, going, in the first instance, to peer review. Reviews that have not entered under this procedure will not be considered.
Special articles may be requested by the Editorial Board or referred at the initiative of the authors. In any case, the Editorial Board of the RMU reserves the absolute power to evaluate, reject, assign the category it deems appropriate and request the modifications that are considered pertinent to the authors, who may or may not endorse the aforementioned modifications, remaining at consideration of the Editorial Board the final approval for the publication of the article. Articles may have an extension of up to ten pages, double spaced, font size 12 and a maximum of 30 bibliographical citations. They may not have more than five figures or tables.
- Editorial Comment Section
This section is intended for comments on articles published in the RMU, in the same issue or in previous issues, or on issues that are under discussion or controversy. Its objective is to put the subject of the article in context, point out the main contributions, uncertainties and areas in which the investigation must deepen. They will be requested by the Editorial Committee of the RMU. The maximum length will be two double-spaced pages with a table or figure and, if applicable, up to five bibliographical citations.
- Technique Update Section
Its maximum length will be six double-spaced pages, font size 12, no more than six figures or tables will be included.
- Letters to the Editor Section
Includes communications, observations, comments on issues related to medical work or the RMU. The maximum length will be three double-spaced pages with a table or figure and, if applicable, up to five bibliographical citations. It must be in letter format, addressed to the Director of the Uruguayan Medical Journal; the maximum number of signatories will be four. The affiliation of the authors will be specified synthetically. It may have a title with a maximum length of 10 words.
- Imaging in medicine
This section is intended for the publication of images that stand out for their formative value. The image must be sent in JPEG or TIFF format and have a quality of no less than 600 dots per inch. It must be accompanied by a text with a maximum length of 200 words and may include up to 2 bibliographical references. No more than 4 authors should appear.
- Scientific videos
This section is intended for the publication of videos that stand out for their educational value.
Opening title
You must include the title, authors of the work, and affiliations at the beginning of the video, either as a title photo or as a text overlay. Be sure to leave the title long enough for it to read (up to 10 seconds for Video Figures and up to 4 seconds for Video Previews).
Third party material and copyright
The authors retain copyright to the videos, but RMU requires you to sign an agreement allowing RMU to distribute the material.
It is very important that you have the rights to use all the material that is included in your presentation, including music, video, images, etc. Permission to use video, audio, or images of identifiable individuals or proprietary content rests with the author. , not in the RMU. We encourage you to use Creative Commons content, for example, music available on ccMixter or Newgrounds. If you need to use copyrighted work, you must cite the source and indicate that your work is not for profit. Take into account if your video is on a youtube channel that you should be aware of the information provided by the platform on copyright regarding the reuse of third-party material https://support.google.com/youtube/answer/2797466#zippy =%2Cqu%C3%A9-types-of-works-is%C3%A1n-subject-to-copyright.
Along with the video, a written summary must be sent, with a maximum length of 150 words that will include an introduction, followed by the description of the contents of the video as well as the pertinent comments.
In the case of a clinical case, it must be specified in the text and at the bottom of the video that the approval of the patient and/or family member responsible for the dissemination of the clinical case has been obtained.
It will have a maximum duration of 3 minutes and must have a voiceover in Spanish.
No more than 5 authors may appear per-video.
They must be edited in formats used by the YouTube platform.
The material sent must not include promotions or commercial advertisements.
- Review of scientific articles, books and magazines
In this section there are works that are comments on magazine articles and books
For additional information and advice for the presentation of the articles, the authors can contact the Editorial Board of the RMU: secretariarmu@gmail.com
* Requires endorsement from the Editorial Board to be sent.
# At the discretion of the Editorial Board.
[1] Unique identifier that gives researchers a number that allows them to compile their research production. ORCID: Open Researcher and Contributor ID. Available at: https://orcid.org
[2] Available online: https://www.impo.com.uy/bases/decretos-originales/158-2019
[3] Unique identifier that gives researchers a number that allows you to compile your investigative production. ORCID: Open Researcher and Contributor ID. Available at: https://orcid.org
Comentario de artículos científicos, libros y revistas
In this section there are works that are comments on journal articles and books
Privacy Statement
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.











